Abstract
The fusion protein PML-RARA initiates acute promyelocytic leukemia (APL). Combination therapy with ATRA and arsenic trioxide cures the vast majority of APL patients, however the underlying molecular mechanism(s) by which PML-RARA initiates APL are not yet clear. PML-RARA is thought to act as a transcription factor to regulate the expression of target genes. Currently, however, there is essentially no consensus regarding the binding sites of PML-RARA in the genomes of early myeloid cells and APL cells - only 6 binding sites were shared among the 3 previous studies1-3. We recently found that the ability of PML-RARA to initiate aberrant serial replating (a surrogate for self-renewal) is dependent upon its ability to bind its DNA targets, since PML-RARA with a point mutation (C88A) in the DNA binding domain of RARA prevents PML-RARA-induced serial replating4. Therefore, PML-RARA may initiate APL by binding to specific DNA sequences in the genomes of hematopoietic cells, which may dysregulate the expression of target gene(s) that are essential for 'reprogramming’ these cells to self-renew.
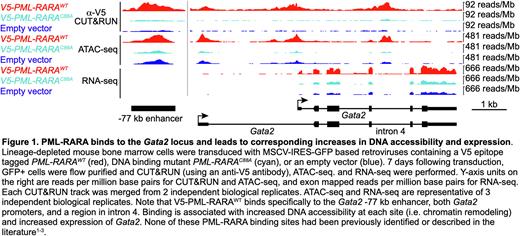
Since high quality antibodies that specifically detect PML-RARA fusion proteins do not currently exist, we generated V5 epitope tagged PML-RARA retroviral constructs. We verified that the V5 tag does not disrupt PML-RARA function, since V5-PML-RARA retains its ability to induce serial replating in colony forming assays, and it disrupts PML oncogenic domains (PODs). We transduced lineage-depleted mouse bone marrow cells (enriched for hematopoietic stem/progenitor cells; HSPCs) with MSCV-IRES-GFP based retroviruses containing V5-PML-RARAWT, V5-PML-RARAC88A, or an empty vector. 7 days after transduction, GFP+ cells were flow purified, and anti-V5 ChIP-seq and CUT&RUN (as well as single cell RNA-seq and ATAC-seq) were performed. ChIP-seq and CUT&RUN identified 2,958 genomic PML-RARA binding sites that were within 10 kb of 3,119 genes (significance determined by MACS3.0 software) in WT HSPC genomes. Of these, 67 binding sites were associated with concurrent changes in both DNA accessibility (defined by ATAC-seq) and expression by scRNA-seq (fold change ³ 2 and FDR £ 0.05 by ANOVA), suggesting that they are direct target genes (example in Figure 1). For orthogonal validation, we performed ATAC-seq and bulk RNA-seq in sorted promyelocytes from pre-leukemic Ctsg-PML-RARA knock-in mice (compared to WT littermates); this approach showed similar results.
To extend our studies to primary human HSPCs, we performed scRNA-seq and anti-V5 ChIP-seq in CD34+ cord blood cells after retroviral transduction with V5-PML-RARA. This analysis identified 1,766 V5-PML-RARA ChIP-seq binding sites, of which only 3 were concordant with previously identified sites1-3. Integration of our scRNA-seq and ChIP-seq data revealed that 14 genes were bound and coordinately dysregulated by PML-RARA in both human and mouse HSPCs. These PML-RARA regulated genes include GATA2, RUNX1, SOCS2, and SPI1/PU.1, all of which have previously been implicated in the pathogenesis of acute myeloid leukemia or APL. Only 3 of the 2,958 binding sites identified were concordant with the previously published sites1-3. Finally, our analysis also revealed that 224 genes are coordinately dysregulated (127 up, 97 down) following PML-RARA expression in both human and mouse HSPCs by scRNA-seq, but do not have PML-RARA binding sites within 10 kb of those genes - thereby suggesting that they are indirectly influenced by PML-RARA. These data have allowed us to identify several genes that may be directly regulated by PML-RARA binding, leading to chromatin remodeling, and ultimately to changes in gene expression. These data may lead to the identification of common molecular mechanisms utilized by PML-RARA (and other AML initiating mutations) to reprogram HSPCs, making them more 'fit’ for transformation.
References: 1. Martens JH, Brinkman AB, Simmer F, et al. Cancer Cell. 2010;17(2):173-85.
2. Wang K, Wang P, Shi J, et al. Cancer Cell. 2010;17(2):186-97.
3. Saeed S, Logie C, Stunnenberg HG, Martens JH. Br J Cancer. 2011;104(4):554-8.
4. Katerndahl CDS, Rogers ORS, Day RB, et al. Blood. 2021;138(13):1148-1161.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal